Oncology Program: Breast Cancer
Enobosarm
Estrogen is one of the main drivers of breast cancer proliferation, tumor progression, and metastasis. Up to 85% of breast cancers are ER+, and consequently, estrogen is one of the main drivers of breast cancer proliferation, tumor progression, and metastasis. Consequently, treatments that target the estrogen receptor (ER) have been the mainstay of breast cancer therapy, but unfortunately breast cancers in almost all women will eventually develop resistance to endocrine therapies with tumor progression, and alternative treatment approaches will be required including IV chemotherapy.
Targeting the AR has the potential to be the next important endocrine therapy for women with breast cancer. AR is the most abundantly expressed steroid receptor in breast cancer being detected in between 70 to 95% of breast cancer specimens, and androgen receptor agonists inhibit cellular proliferation and have antitumor efficacy in ER+ human breast cancer models.
Enobosarm is a new class of endocrine therapy for advanced breast cancer. Enobosarm is an oral, new chemical entity, selective androgen receptor modulator designed to activate the AR in AR+ ER+ HER2- metastatic breast cancer and thereby suppress tumor growth without the unwanted masculinizing side effects. Enobosarm has extensive nonclinical and clinical experience having been evaluated in 27 separate clinical studies in approximately 1,580 subjects dosed, including three Phase 2 clinical trials in advanced breast cancer involving more than 250 patients. In one of the Phase 2 clinical trials conducted in women with AR+ ER+ HER2- metastatic breast cancer, enobosarm demonstrated significant antitumor efficacy in heavily pretreated cohorts that failed estrogen blocking agents, chemotherapy and/or CDK 4/6 inhibitors and was well tolerated with a favorable safety profile.
The current standard of care for first line treatment of ER+ HER2- metastatic breast cancer is treatment with a CDK 4/6 inhibitor in combination with an estrogen blocking agent. Once a patient progresses while receiving this combination therapy, the FDA-approved treatment choices are limited to another estrogen blocking agent or chemotherapy. As up to 95% of ER+ HER2- metastatic breast cancers have an androgen receptor, we are developing enobosarm as another, but different, hormone therapy for the second line treatment of ER+ HER2- metastatic breast cancer. In preclinical studies, metastatic breast cancer tissue samples taken from patients who have ER+ HER2- metastatic breast cancer that had become resistant to CDK 4/6 inhibitors and estrogen blocking agents were grown in mice. In these mice, treatment with enobosarm in combination with a CDK 4/6 inhibitor suppressed the growth of human metastatic breast cancer greater than the CDK 4/6 inhibitor alone. Further, enobosarm treatment alone was also effective in suppressing the growth of CDK 4/6 inhibitor and estrogen blocking agent resistant human metastatic breast cancer tumors in mice.
The Phase 2 clinical trial (G200802) was a 2-arm study evaluating 9mg and 18mg enobosarm daily oral dosing in 136 women with AR+ ER+ HER2- metastatic breast cancer. The patients in this study were also heavily pretreated having failed an average of 3.7 endocrine treatments, 90% had received prior chemotherapy, and 12% had prior treatment with CDK4/6 inhibitor. Enobosarm showed efficacy with a clinical benefit rate (CBR) at 6 months which for 9mg was 32% (95% CI 19.5%,46.7%) and for the 18mg cohort was 29% (95% CI 17.1%,43.1%). The median duration of clinical benefit was not reached for the 9mg group (8.2 month – Not reached) and for the 18mg group was 14.1 months (11 months – 16.5 months). A post-hoc androgen receptor expression subset analysis using the androgen receptor testing measure used in G200802 was also performed in this population with known androgen receptor status and measurable disease (n=84). Objective tumor responses correlated with the degree of percent androgen receptor staining. Using a 40% androgen receptor staining cutoff, clinical benefit at 24 weeks for ≥40% androgen receptor was 52% and <40% androgen receptor was 14% (p<0.0004). Overall response rate in subjects with ≥40% androgen receptor staining was 34% and <40% androgen receptor was 2.7% (p=0.0003). Median progression free survival (PFS) for ≥40% androgen receptor was 5.47 months (95% CI 2.83-11.13) versus <40% androgen receptor was 2.73 months (95% CI 2.63 – 2.80) (p<0.001). Enobosarm treatment was well tolerated with significant positive effects on quality-of-life measurements. The 9 mg group had a slightly better safety profile than the 18 mg group.
In summary, treatment with enobosarm, a novel oral selective androgen receptor modulator, resulted in clinically significant objective tumor responses, improvement in quality of life, and favorable safety profile in a heavily pretreated population of women with AR+ER-HER2- metastatic breast cancer. Higher percent androgen receptor nuclei staining correlated with a greater antitumor activity. By targeting and activating AR in breast cancer tumors with sufficient androgen receptor expression, women with metastatic breast cancer may be identified who are most likely to respond to enobosarm therapy. Overall, these studies of enobosarm clearly establish the clinical relevance of targeting the androgen receptor with a selective androgen receptor agonist. Enobosarm introduces a novel endocrine therapy to patients with breast cancer that have exhausted endocrine therapies targeting estrogen receptor, but prior to intravenous chemotherapy.
On March 30, 2023 and November 3, 2023, we met with the FDA to discuss the design of our Phase 3 clinical trial in patients with AR+ ER+ HER2- metastatic breast cancer who have tumor progression while receiving palbociclib (a CDK 4/6 inhibitor) plus an estrogen blocking agent (nonsteroidal aromatase inhibitor or selective estrogen receptor degrader). The design of the Phase 3 clinical trial was amended following our November 3, 2023 meeting with the FDA to implement the recommendations that were provided by the FDA.
A diagram of the Phase 3 clinical trial is shown in the figure below:
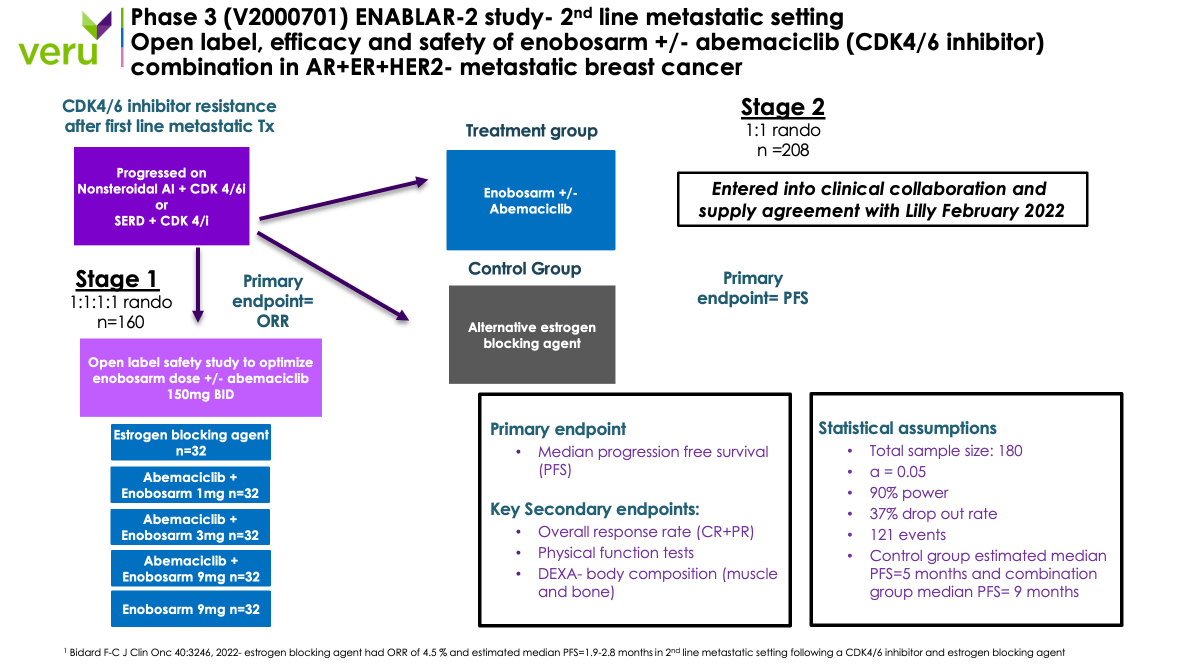
The design of our Phase 3 clinical trial is evaluating enobosarm alone or in combination with abemaciclib, a CDK 4/6 inhibitor, in patients with AR+ ER+ HER2- metastatic breast cancer who have tumor progression while receiving palbociclib (a CDK 4/6 inhibitor) plus an estrogen blocking agent. The primary endpoint for the Stage 1 portion of the Phase 3 clinical trial is objective tumor response rates (“ORR”). As of August 2023, we had completed the target enrollment of three patients in the Stage 1a portion of the Phase 3 clinical trial to assess the safety and pharmacokinetics of the combination of abemaciclib and enobosarm. There were no reported drug-to-drug interactions between abemaciclib and enobosarm or new safety findings in the three patients. Further, the early preliminary clinical results showed two partial responses and one stable disease in the first three patients based on local assessments, and as of the cutoff date the patients were on study for 9, 11 and 12 months from first day of dosing to disease progression by blinded central assessment.
As we have prioritized our clinical programs to focus on enobosarm for high quality weight loss, the continued clinical development of enobosarm for the treatment of metastatic breast cancer is subject to the availability of sufficient funding.

