Obesity and Overweight Program
Enobosarm
According to the CDC, 41.5% of older adults have obesity in the United States and could benefit from a weight loss medication. Up to 34.4% of these obese patients over the age of 60 have sarcopenic obesity which means patients are overweight or obese and have age-related low muscle mass. Sarcopenic obese patients are potentially at the greatest risk for developing critically low amounts of muscle mass when taking a GLP-1 RA medication for the treatment of obesity. Patients with critically low muscle mass may experience muscle weakness leading to poor balance, decreased gait speed, mobility disability, loss of independence, falls, bone fractures and increased mortality. We believe there is an urgent unmet medical need for a drug when given in combination with a GLP-1 RA that could prevent the loss of muscle, while preferentially reducing fat in not only overweight or obese patients, but especially for sarcopenic obese or overweight elderly patients who are at-risk for developing muscle atrophy and muscle weakness leading to frailty. We believe that enobosarm, our novel small molecule, oral selective androgen receptor modulator, may be the best drug candidate to address this unmet medical need.
These clinical trials include two Phase 2 clinical trials in 168 healthy older or sarcopenic subjects and one Phase 2b clinical trial and two Phase 3 clinical trials in 800 subjects who have muscle loss caused by cancer. The totality of the clinical data from these five clinical trials demonstrates that enobosarm treatment leads to dose-dependent increases in muscle mass with improvements in physical function as well as significant dose-dependent reductions in fat mass. Although these 5 clinical trials were not specifically conducted in an obese population, an ad hoc subset analysis was performed on obese patients, who had a BMI of 30 or greater, who were enrolled in the Phase 3 placebo-controlled 504 clinical study which evaluated enobosarm 3mg treatment in metastatic lung cancer patients on chemotherapy. Even though a small sample size of 29 subjects, notable differences consistent with an obesity drug that preserves muscle and decreases fat were observed. At 12 weeks, enobosarm 3mg treated subjects had 4.96% increase in total lean body mass (muscle) compared to placebo and a 5.77 % reduction in fat mass compared to placebo. By 21 weeks, enobosarm 3mg treatment resulted in a 14.4% loss in total fat mass and a 4.51% loss of total DEXA body weight compared to placebo while maintaining total lean mass. It should be noted that these results were from the short-term treatment of enobosarm alone. The expectation is that enobosarm in combination with a GLP-1 RA would potentially augment the fat reduction and weight loss while avoiding muscle loss.
In the Phase 2 clinical trial evaluating enobosarm in 120 men over 60 years old and postmenopausal women treated for 12 weeks, patients receiving 3mg dose of enobosarm (n=24) demonstrated a statistically significant (i) increase in total lean body mass (average increase of 1.25 kg (p = < 0.001)) and (ii) decrease in total fat mass (average decrease of 0.32 kg (p=0.049)). When measuring physical function by stair climb test, patients receiving 3mg dose of enobosarm in this trial also demonstrated statistically significant improvements compared to placebo (p=0.049).
In addition, enobosarm has a large safety database, which includes 27 clinical trials involving 1581 men and women dosed with duration of treatment in some patients for up to 3 years. In this large safety database, enobosarm was generally well tolerated with no increase in gastrointestinal side effects. This is important as there are already significant and frequent gastrointestinal side effects with a GLP-1 RA treatment alone.
Although these 5 studies were previously conducted by GTx or Merck, Veru owns all this clinical data as part of our enobosarm exclusive global in-license agreement.
Because of these key clinical attributes, we believe that enobosarm may address this unmet medical need. The patient data that were generated from these five enobosarm clinical trials in both elderly patients and in patients with a cancer induced starvation-like state provide strong clinical rationale for enobosarm to address two possible populations:
The Phase 2b, multicenter, double-blind, placebo-controlled, randomized, dose-finding clinical trial is designed to evaluate the safety and efficacy of enobosarm 3mg, enobosarm 6mg, or placebo as a treatment to augment fat loss and to prevent muscle loss in 90 sarcopenic obese or overweight elderly patients receiving a GLP-1 RA who are at-risk for developing muscle atrophy and muscle weakness. The primary endpoint is lean body mass (muscle), and the key secondary endpoint is total body fat mass at 16 weeks. The clinical study enrolled its first patient in April 2024 with the topline clinical results from the trial expected in the end of the fourth calendar quarter of 2024.
A diagram of the Phase 2b clinical trial design is shown in the figure below.
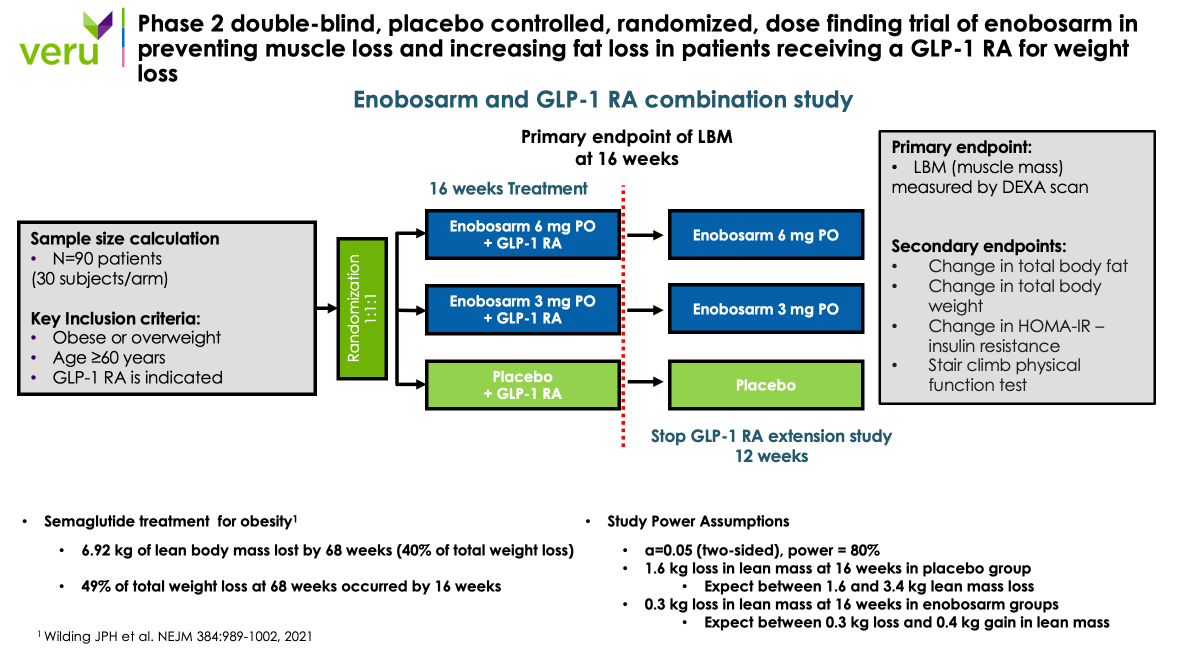
After completing the efficacy dose-finding portion of the Phase 2b clinical trial, participants will then continue into a Phase 2b extension clinical trial where all patients will stop receiving a GLP-1 RA, but will continue taking placebo, enobosarm 3mg, or enobosarm 6 mg for an additional 12 weeks. The Phase 2b extension clinical trial will evaluate whether enobosarm can maintain muscle and prevent the fat and weight rebound that occurs after stopping a GLP-1 RA drug. The results of the separate Phase 2b extension clinical study is expected in calendar Q2 2025.
In the US, 42% of older adults (> 60 years of age) have obesity (CDC) and 34% of these patients also have sarcopenia, or low muscle reserve. Accordingly, enobosarm is targeting at risk older obese or overweight patients who may already have low muscle mass, also known as sarcopenic obesity, and the further drop in muscle mass of all-important muscles increases risk of muscle weakness, functional limitations, mobility disability, falls, higher hospitalizations, and greater mortality. It should be emphasized that enobosarm may potentially be combined with any one of the many GLP-1 RA weight loss drugs, not only for older or overweight at-risk patients, but also all overweight or obese patients who want to avoid muscle loss when taking a GLP-1 RA for weight loss. The combination of enobosarm with a GLP-1 receptor agonist potentially represents a multi-billion-dollar global opportunity.

