Infectious Disease Program
Sabizabulin for hospitalized patients with mild to severe viral-induced ARDS
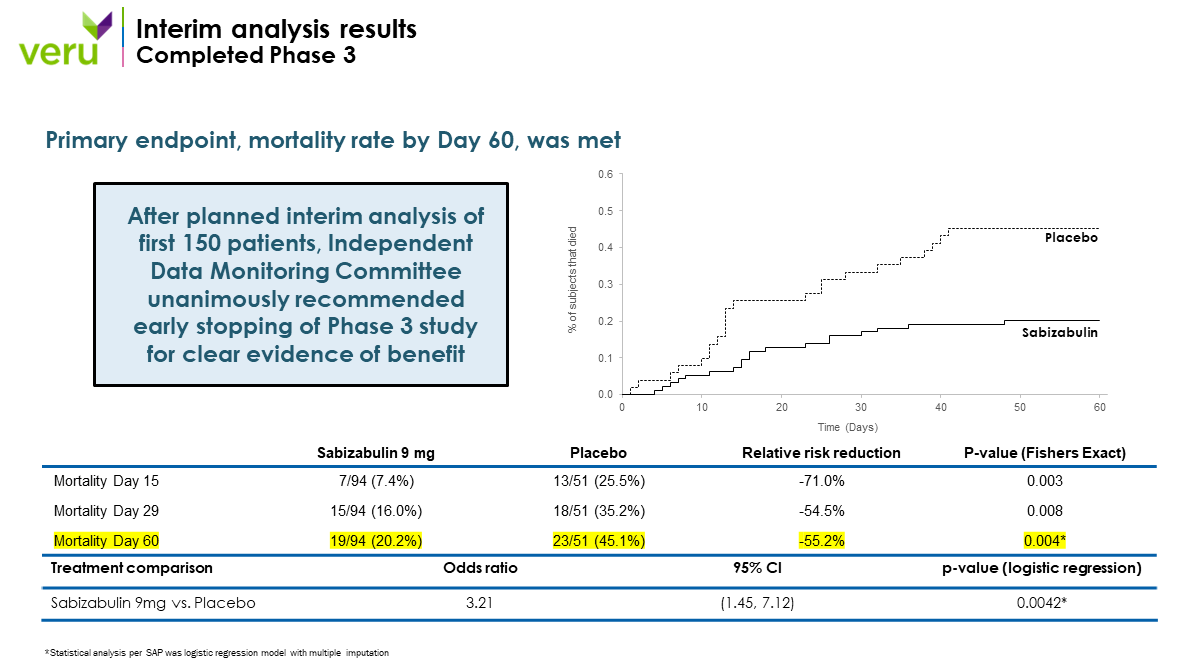
We have completed positive Phase 2 and positive Phase 3 COVID-19 clinical trials, which have demonstrated that sabizabulin treatment resulted in a significant mortality benefit in hospitalized moderate to severe patients with COVID-19 viral lung infection at high risk for ARDS and death.
Highlights from the positive Phase 3 clinical trial published in New England Journal of Medicine Evidence:
Although in September 2023 we received agreement from the FDA on the design of a Phase 3 clinical trial broadly evaluating sabizabulin in any viral-induced ARDS, we will continue to seek external funding through government grants, pharmaceutical partnerships, and similar sources to fund the clinical development program. Without such external funding, we do not plan to advance the Phase 3 development of sabizabulin as a treatment for viral-induced ARDS.

