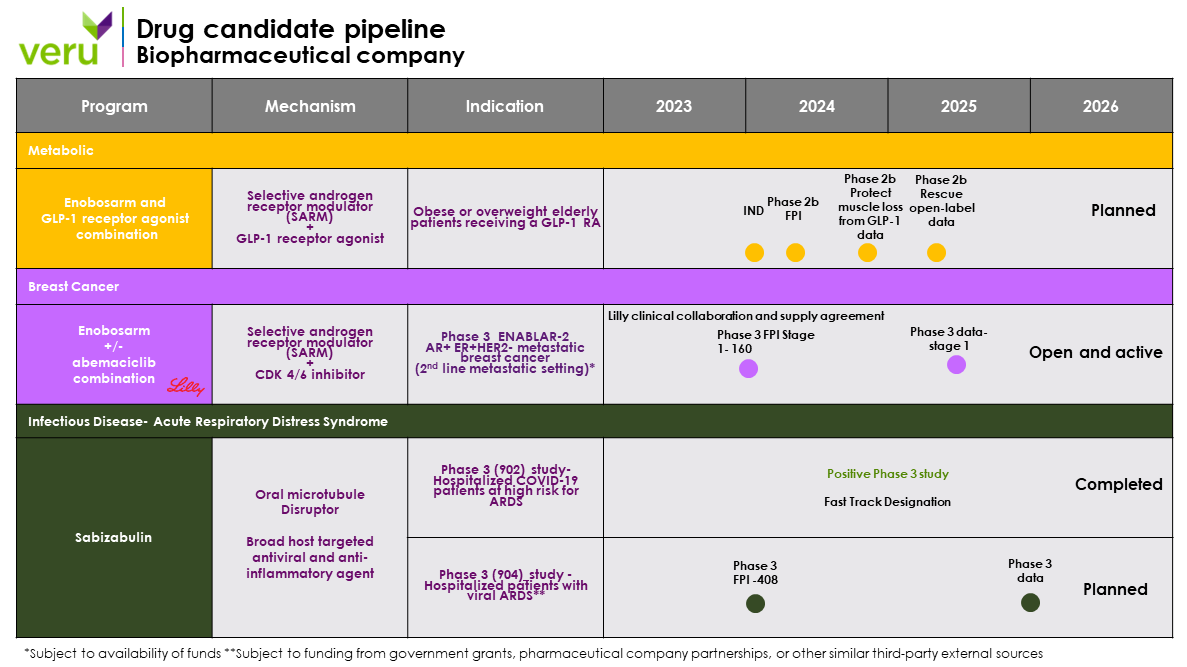
Pipeline
We are a late clinical stage biopharmaceutical company focused on developing novel medicines for high-quality weight loss, oncology, and viral ARDS.

Drug Development Program
Our drug development program includes two late-stage new chemical entities, enobosarm and sabizabulin. Enobosarm (aka ostarine, MK-2866, GTx-024, S-22, and VERU-024), an oral selective androgen receptor modulator (SARM), is being developed as a treatment in combination with weight loss drugs to augment fat loss and to avoid muscle loss in overweight or obese patients for chronic weight management. Initially, enobosarm will be developed in a Phase 2b clinical study to address the large subpopulation of sarcopenic obese or overweight elderly patients receiving a GLP-1 RA who are at-risk for developing muscle atrophy and muscle weakness leading to physical function mobility disability and frailty and (ii) enobosarm for the treatment of androgen receptor positive (AR+), estrogen receptor positive (ER+) and human epidermal growth factor receptor 2 negative (HER2-) metastatic breast cancer in the 2nd line setting. Sabizabulin, a microtubule disruptor, is being developed for the treatment of hospitalized patients with viral-induced ARDS. We also have an FDA-approved commercial product, the FC2 Female Condom® (Internal Condom), for the dual protection against unplanned pregnancy and sexually transmitted infections.